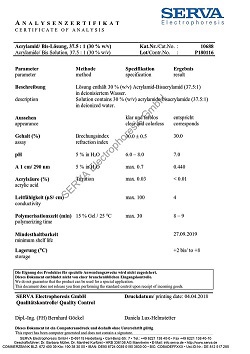
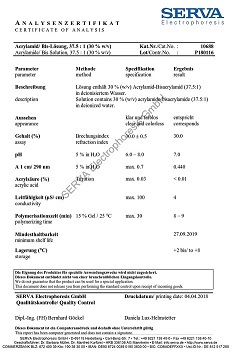
Dodecyl-ß-D-maltoside
The sugar surfactant dodecyl-ß-D-maltoside is an efficient membrane solubilizer. It has frequently been used for the investigation of photosynthetic membranes, especially for the solubilization of highly active photosystem II particles.
Dodecyl-ß-D-maltoside has also successfully been used for the isolation and characterization of membrane bound enzymes, receptors and other protein complexes, e.g. for the solubilization of the muscarinergic acetylcholine receptor, the stabilization of Ca2+-ATPase or the reconstitution of a cytochrome b1 complex.
The dodecyl maltoside-SDS 2D-PAGE is a powerful method for the separation of chloro¬plast thylakoid membrane proteins.
Synonyms: Dodecyl-ß-D-maltopyranoside, Lauryl maltoside
CAS registry number: [69227-93-6]
Molecular formula: C24H46O11
Relative molecular mass (Mr): 510.6
Classification: Non-ionic surfactant
Literature specification:
• Critical miceilar concentration (CMC): 0.15 mM
• Aggregation number (Na): 98
Bibliography
Solubilization and Characterization of Photosystems
Bass, W.T., Bricker, T.M. (1988) Dodecyl maltoside-sodium dodecyl sulfate two-dimensional polyacrylamide gel electrophoresis of chloroplast thylakoid membrane proteins. Anal. Bio-chem. 171, 330-8.
Bendall, D.S., Bowes, J.M., Stewart, A.C. a. Taylor, M.E. (1988) Oxygen-evolving photosystem II particles from Phormidium laminosum. Methods Enzymol. 167, 272-80.
Dekker, J.P., Boekema, E.J., Witt, H.T., Roegner, M. (1988) Refined purification and further characterization of oxygen-evolving and Tris-treated photosystem II particles from the thermophilic cyanobacterium Synechococcus sp.. Biochim. Biophys. Acta 936, 307-18.
Dekkcr, J.P., Bowlby, N.R. a. Yocum, C.F. (1989) Chlorophyll and cytochrome b-559 content of the photochemical reaction center of photosystem II. FEBS Lett. 254, 150-4.
Hodges, M. a. Moya, I. (1988) Time-resolved chlorophyll fluorescence studies on pigment-protein complexes from photosynthetic membranes. Biochim. Biophys. Acta 935, 41-52.
Van Wijk, K.J. et al. (1995) In vitro synthesis and assembly of photosystem II core proteins. The D1 protein can be incorporated into photosystem II in isolated chloroplasts and thylakoids. J. Biol. Chem. 270, 25685-95.
Eshagi, S. et al. (2000) Functional characterization of the PS II-LHC II supercomplex isolated by a direct method from spinach thylakoid membranes. Potosynth. Res. 64, 179-87.
Bergantino, E. et al. (2003) Light- and pH-dependent structural changes in the PsbS subunit of photosystem II. PNAS 100, 15265-70.
Solubilization and Characterization of Enzymes
Tandon, S. a. Horowitz, P. (1988) The effects of lauryl maltoside on the reactivation of several enzymes after treatment with guanidinium chloride. Biochim. Biophys. Acta 955, 19-25.
Yang, X.. a. Trumpower, B.L. (1988) Protonmotive Q cycle pathway of electron transfer and en¬ergy transduction in the three-subunit ubiqui¬nol-cytochrome c oxidoreductase complex of Paracoccus denitrificans. J. Biol. Chem. 263, 11962-70.
De Foresta, B., Le Maire, M., Orlowski, S., Champeil, P., Lund, S., Moeller, J.V., Michelangeli, F. a. Lee, A.G. (1989) Membrane solubilization by detergent: use of brominated phospholipids to evaluate the detergent-induced changes in calcium-ATPase/lipid interaction. Biochemistry 28, 2558-67.
Lund, S., Orlowski, S., De Foresta, B., Champeil, P., Le Maire, M. a. Moeller, J. V. (1989) Detergent structure and associated lipid as determinants in the stabilization of solubilized calcium ATPase from sarcoplasmic reticulum. J. Biol. Chem. 264, 4907-15.
Smith, D., Drummond, S. a. Dalton, H. (1989) Solubilization of methane monooxygenase from Methylococcus capsulatus (Bath). Eur.J I. Biochem. 182, 667-71.
Musatov, A., Permyakov, E.A., Bagelova, J., Morozova, L. a. Shnyrov, V.L. (1990) Some aspects of fluorescence and microcalorimetric studies of cytochrome c oxidase. Biochem. Int.21, 563-71.
James, S.R. et al. (1995) Kinetic analysis of phospholipase C beta isoforms using phospholipid-detergent mixed micelles. Evidence for interfacial catalysis involving distinct micelle binding and catalytic steps. J. Biol. Chem. 270, 11872-81.
Bonza, C. et al. (1998) Purification of the plasma membrane Ca2+-ATPase from radish seedlings by calmodulin-agarose affinity chromatography. Plant Physiol. 116, 845-51.
Hettmann, T. et al. (1998) Cytochrome b558/566 from the archaeon Sulfolobus avidocaldarius. A novel highly glycosylated membrane-bound b-type hemoprotein. J. Biol. Chem. 273, 12032-40.
Olivari, C. et al. (1998) Fusicoccin Binding to its plasma membrane receptor and the activation of the plasma membrane H+-ATPase. IV. Fusicoccin induces the association between the plasma membrane H+-ATPase and the Fusicoccin receptor. Plant Physiol. 116, 529-37.
Al-Dabbagh, B. et al. (2008) Purification and characterization of the bacterial UDP-GlcNAc:undecaprenyl-phosphate GlcNAc-1-phosphate transferase WecA. J. Bacteriol. 190, 7141-6.
Solubilization and Characterization of Receptors
Peterson, G.L., Rosenbaum, L.C. a. Schimerlik, M. I. (1988) Solubilization and hydrodynamic properties of pig atrial muscari¬nic acetylcholine receptor in dodecyl-ß-D- mal¬toside. Biochem. J. 255, 553-60.
Marquer, C. et al. (2001) Influence of MT7 toxin n the oligomerization state of the M1 muscarinic receptor. Biol. Cell 102, 409-20.
Schiller, H. et al. (2001) Solubilization and purification of the human ETB endothelin receptor produced by high-level fermentation in Pichia pastoris. Receptors Channels 7, 453-69.
Navratilova, I. Et al. (2005) Solubilization, stabilization, and purification of chemokine receptors using biosensor technology. Anal. Biochem. 339, 271-81.
Jastrzebska, B. et al. (2006) Functional and structural characterization of rhodopsin oligomers. J. Biol. Chem. 281, 11917-22.
Leitz, A.J. Et al. (2006) Functional reconstitutionof ß2-adrenergic receptors utilizing self-assembling nanodisc technology.BioTechniques 40, 601-12.
Du, A.T. et al. (2010) Ligand-supported purification of the urotensin-II receptor. Molec. Pharmacol. 78, 639-47.
Investigation of other Proteins/Protein Complexes
Timmins, P.A., Leonhard, M., Weltzien, H.U., Wacker, T. a.Welte, W. (1988) A physical charac¬terization of some detergents of potential use for membrane protein crystallization. FEBS Lett 238, 361-8.
Koenig, B., Welte, W. a. Hofmann, K.P. (1989) Photoactivation of rhodopsin and interaction with transducin in detergent micelles. Effect of 'doping' with steroid molecules. FEBS Lett. 257,163-6.
Sedzik, J. a. Tsukihara, T. (2000) Solubilization of PNS myelin membrane proteins by detergents. Neuroreport 11, 2559-63.
Carroll, J. et al. (2002) Definition of the nuclear encoded protein composition of bovine heart mitochondrial complex I. Identification of two new subunits. J. Biol. Chem. 277, 50311-7.
Luche, S. et al. (2003) Evaluation of nonionic and zwitterionic detergents as membrane proteins solubilizers in two-dimenssional electrophoresis. Proteomics 3, 249-53.
Griffiths, L.G. et al. (2008) Protein extraction and 2-DE of water- and lipid-soluble proteins from bovine pericardium, a low-cellularity tissue. Electrophoresis 29, 4508-15
Paul, V. et al. (2008) A novel enzyme immunoassay specific for ABCA1 protein quantification in human tissues and cells. J. Lipid Res. 49, 2259-67.
Sasaki, T. et al. (2011) Sensitive detection of protein-lipid interaction change on bacteriorhodopsin using dodecyl ß-D-maltoside. Biochemistry 50, 2283-90.
Further Applications
Manzella, S.M. et al. (1995) Dodecyl-beta-D-maltoside as a substrate for glucosyl and xylosyl transfer by glycogenin. Glycobiology 5, 263-71.
Bae, W.S. a. Urban, M.W. (2006) Lectin-recognizable colloidal dispersions stabilized by n-dodecyl beta-D-maltoside: particle-particle and particle-surface interactions. Biomacromolecules 7, 1156-61.
Dang, F. et al. (2006) Hybrid dynamic coating with n-dodecyl beta-D-maltoside and methyl cellulose for high-performance carbohydrate analysis on poly(methyl methacryllate) chips. Anla. Chem. 78, 1452-8
Lu, S. et al. (2007) pH dependence of adsorption of n-dodecyl-ß-D-maltoside on solids. J. Colloid Interface Sci. 316, 310-6.