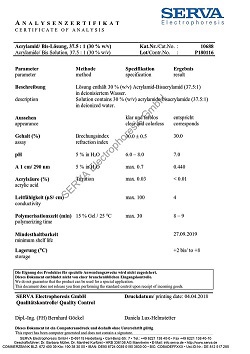
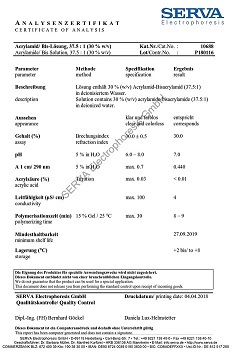
Saponin from Quillaja bark
Saponins are a group of gycosides which occur in many plant families but also in the animal class of echinodermata, e.g. star fish and see cucumber. They are classified according to the nature of their aglycon (sapogenin) in 2 major groups: steroid (C-27 structure), and triterpene (C-30 structure) saponins. Most saponins belong to the latter type which occurs mainly in dicotyledonous plants, while steroid saponins mainly occur in monocotyledons.
The Quillaja saponin is a triterpene saponin isolated from the bark of the soap tree (Quillaja saponaria, belonging to the Rosaceae family), which occurs in Bolivia, Chile and Peru. The name Panama bark does not refer to the origin of the plant but to the former place of exportation. The product is a heterogeneous mixture of molecules varying both in their sapogenin and sugar moieties. The main aglycon moiety is quillaic acid, a pentacyclic triterpene of the 18 α-oleanane type.
Saponins are characterized by their ability to form in water colloidal soap-like solutions. Such solutions are not very stable, must not be autoclaved and can be stored at 4 °C for maximally 3 days. It is recommended to always use freshly prepared solutions. Saponins show haemolytic activity and are toxic for fishes.
They influence membrane permeability by forming complexes with cholesterol.
Saponin solutions are used to permeabilize cells; it can form pores in the membrane by which - after fixation – fluorescent dyes, antibodies or other compounds can be imported into the cell.
Saponins can also be used for stimulation of immune response.
Synonym: Sapogenin glycoside
CAS registry number: [8047-15-2]
Classification: Triterpene saponin of the Oleanane type
BIBLIOGRAPHY
Interaction with Membrane Compounds
Bergman, A.S. and Carlsson, S.R. (1994) Saponin-induced release of cell-surface-anchored Thy-1 by serum glycosylphosphatidylinositol-specific phospholipase D. Biochem. J. 298,661-8.
Baumann, E. et al. (2000) Hemolysis of human erythrocytes with saponin affects the membrane structure. Acta Histochemica 102, 21-35
Mitra, S. a. Dungan, S.R. (2000) Micellar properties of quillaja saponin.2. Effect of solubilized cholesterol on solution properties. Colloids a. Surfaces 17, 117-33.
Mitra, S. a. Dungan, S.R. (2001) Cholesterol solubilization in aqueous micellar solutions of quillaja saponin, bile salts or non-ionic surfactants. J. Agric. Food Chem. 49, 384-94
Permeabilization of Cell Membranes
Hedman, K. (1980) Intracellular localization of fibronectin using immunoperoxidase cytochemistry in light and electron microscopy. J. Histochem. Cytochem. 28, 1233-41
Wassler, M. et al. (1987) Differential permeabilization of membranes by saponin treatment of isolated rat hepatocytes. Biochem. J. 247, 407-15.
Jacob, M.C. et al. (1991) Membrane cell permeabilization with saponin and multiparametric analysis by flow cytometry. Cytometry 12, 550-8
Johnson, J.A. et al. (1996) An improved permeabilization protocol for the introduction of peptides into cardiac myocytes. Circulation Research 79, 1086-96.
Stimulation of Immune Response
Gebara, V.C. et al. (1995) Effect of saponin from Quillaja saponaria (Molina) on antibody, tumor necrosis factor and interferon-gamma production. Biotechnol. Appl. Biochem. 22, 31-7.
Behboudi, S. et al. (1999) Quillaja saponin formulations that stimulate proinflammatory cytokines elicit a potent acquired cell-mediated immunity. Scand. J. Immunol. 50, 371-7
Hu, K.-F. et al. (2001) Different respiratory syncytial virus and Quillaja saponin formulations induce murine peritoneal cells to express different proinflammatory profiles. FEEMS Immunology a. Medical Microbiology 31, 105-12.
Sun, H.X. et al. (2009) Advances in saponin-based adjuvants. Vaccine 27, 1787-96.
Other Applications
Kimura, H. et al. (1999) Quantitation of RNA polymerase II and its transcription factors in an HeLa cell: little soluble holoenzyme but significant amounts of polymerases attached to the nuclear substructure. Mol. Cell. Biol. 19, 5383-92.
Chamberlaine, L.H. et al. (2001) SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. PNAS 98, 5619-24.
Wiesman, Z. a. Chapagain, B.P. (2003) Laboratory evaluation of natural saponin as a bioactive agent against Aedes aegypti and Culex pipiens. Dengue Bulletin 27, 168-73.
Hassan, S.M. et al. (2008) Effects of quillaja saponin (Qillaja saponaria) on early embryonic zebrafish (Danio rerio) development. Int. J. Toxicol. 27, 273-8.
General Information
McIlroy, R.J.: The Plant Glycosides, chapter IX, Edward Arnold & Co., London 1951.
Römpp Chemie Lexikon 9. Auflage, Georg Thieme Verlag, Stuttgart, New York, 1989-1992.
Yang, C.-R. & Tanaka, O. (eds.) Advances in Plant Glycosides, Chemistry and Biology. Elsevier, Amsterdam 1999.
Merck Index 13th ed. # 8442 (2001).