Preparation of Towbin buffers
Preparation of Towbin Buffers
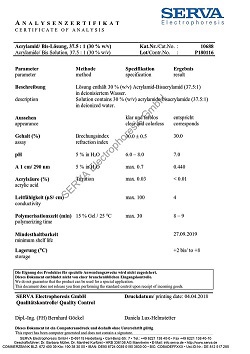
| Buffer 10x | Components | Concentration |
| Tris Glycin pH 8.6 ± 0.2 |
0.25 M 1.92 M |
Working solution:
- Methanol is mostly added to the buffer for achieving efficient binding to the membrane. For nitrocellulose a concentration of ≤20 % (w/v) and for PVDF one of ≤15 % is used. For charged nylon membranes methanol should be omitted from the buffer.
- Methanol may however reduce the solubility of proteins and cause a gel to shrink, which decreases the elution rate. Depending on the sample less or no methanol should be used.
- For native proteins it is recommended to use the buffer without methanol. Addition of 0.1 % SDS (w/v) may improve the elution rate of proteins from an SDS gel. However, SDS reduces the binding of proteins to the membrane. Therefore addition of SDS must be as well determined for each sample individually.
To prepare 1 L 1x running buffer:
To 100 ml 10x concentrate add 200 ml methanol and 700 ml deionized water.
Concentration of components in 1x solution:
• 0.025 M Tris
• 0.192 M Glycine
• 20 % Methanol
Towbin et al., (1979), PNAS USA 76, 4350 – 4354
Gershoni and Palade, (1982), Anal. Biochem. 131, 1 – 15